
UPSIDE Foods
Founded Year
2015Stage
Series C | AliveTotal Raised
$610.85MValuation
$0000Last Raised
$400M | 3 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-67 points in the past 30 days
About UPSIDE Foods
UPSIDE Foods specializes in the cultivated meat within the food industry. The company produces meat grown directly from animal cells and offers a humane and environmentally friendly alternative to traditional meat production. Its cultivated meat is designed to provide the same taste and texture as conventional meat without the need to raise and slaughter animals. It was founded in 2015 and is based in Berkeley, California.
Loading...
ESPs containing UPSIDE Foods
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The cultured meat market, also known as clean meat or cultivated meat, refers to companies that provide a more sustainable source of meat by culturing animal cells. Companies in this market focus on various types of meat, including chicken, pork, fish, and beef. While some of these companies are developing consumer brands, others are focused on building out white-label solutions for manufacturers.…
UPSIDE Foods named as Challenger among 15 other companies, including JUST Egg, Aleph Farms, and Believer Meats.
Loading...
Expert Collections containing UPSIDE Foods
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
UPSIDE Foods is included in 6 Expert Collections, including Agriculture Technology (Agtech).
Agriculture Technology (Agtech)
2,276 items
Companies in the agtech space, such as equipment manufacturers, surveying drones, geospatial intelligence firms, and farm management platforms
Unicorns- Billion Dollar Startups
1,258 items
Food & Beverage
2,802 items
Startups in the food & beverage space, including alternative proteins, vertically-farmed produce, functional beverages and more.
Alternative Proteins
408 items
This Collection includes B2B and B2C companies developing alternatives to animal-derived proteins, including plant-based meat, dairy alternatives, lab-grown or cultured meat, and fermented proteins.
Game Changers 2018
70 items
Wellness Tech
1,370 items
We define wellness tech as companies developing technology to help consumers improve their physical, mental, and social well-being. Companies in this collection play across a wide range of categories, including food and beverage, fitness, personal care, and corporate wellness.
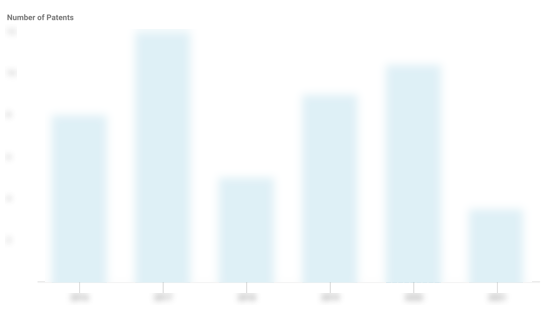
UPSIDE Foods Patents
UPSIDE Foods has filed 60 patents.
The 3 most popular patent topics include:
- biotechnology
- meat industry
- fluid dynamics
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
12/22/2023 | 7/16/2024 | Biotechnology, Clusters of differentiation, Embryology, Transcription factors, Developmental biology | Grant |
Application Date | 12/22/2023 |
|---|---|
Grant Date | 7/16/2024 |
Title | |
Related Topics | Biotechnology, Clusters of differentiation, Embryology, Transcription factors, Developmental biology |
Status | Grant |
Latest UPSIDE Foods News
Dec 21, 2024
npj science of food Abstract Cultivated meat production offers solutions in addressing global food security and sustainability challenges. However, serum-free media (SFM) used in cultivating the cells are expensive, contributing to at least 50% of variable operating costs. This review explores technologies for cost-effective SFM, focusing on reducing cost from using growth factors and recombinant proteins, using affordable raw materials for basal media, and implementing cost-saving measures like media recycling and reducing waste build-up. Introduction With a global population projected to reach 9.6 billion by 2050, the United Nations forecasts a need to increase food production by 70% to meet this demand 1 . Globally, it is also projected that poultry, pork, beef, and lamb consumption will increase by 10–15% by 2032 compared to 2023 levels 2 . Cultivated meat (CM), which requires much less land use compared to traditional farming, thus serves as a promising alternative protein source. CM, produced by growing animal cells in bioreactors, is a growing industry with a market size valued at $221.47 million in 2022 and estimated to reach $592.69 million by 2030 3 . The demand for CM is likely to increase with growing interests in sustainable protein sources for environmental and animal welfare reasons. However, one major obstacle that the industry is currently facing is the high cost of production. The cultivated meat industry is increasingly adopting serum-free media (SFM) to address the cost, ethical, safety, and regulatory concerns associated with using fetal bovine serum (FBS) 4 , 5 , 6 . Currently, SFM makes up at least 50% of variable operating costs in cultivated meat manufacturing 7 , mainly due to the use of growth factors (GFs) and recombinant proteins (RPs) 8 , 9 , 10 , 11 . For example, in the Essential 8 medium, nearly 98% of the media cost can be attributed to FGF-2 and TGF-β 9 . Similarly, the cost of the SFM beefy-9 is largely driven by key components like albumin, FGF-2, and insulin, collectively representing around 60% of the total media cost 10 . Advancements in SFM technology will be major drivers for cultivated meat to reach price parity with conventional meat 9 , 12 , 13 . This review will examine current technologies for developing cost-effective SFM tailored to cultivated meat production, such as reducing cost of growth factors and recombinant proteins, using affordable raw materials for basal media, and optimizing media composition and usage (Fig. 1 ). Fig. 1: Current technologies to reduce the cost of serum-free media (SFM) in cultivated meat production. Strategies include lowering the cost contributions of growth factors, recombinant proteins, and basal media. Additionally, optimizing media components and recycling SFM can enhance media utilization and reduce costs associated with media changes. Methods for serum-free media development This section will explore common methodologies for developing SFM, including substituting FBS with known components, media component screening, omics analysis and systems biology approaches. Firstly, SFM can be developed by substituting FBS with known growth-promoting components, such as growth factors, insulin, transferrin, and selenium. For instance, Mosa Meat developed a SFM by replacing FBS with major and known components of FBS into Ham’s F10 basal medium 8 . Separately, Stout and his team improved upon the reported SFM B8 14 by developing Beefy-9, which adds recombinant albumin and reduces growth-factor concentrations 10 . The Beefy-9 media could support long-term cell culture while retaining myogenic potential 10 . Elsewhere, Skrivergaard’s team used multi-component Design of Experiment (DOE) to screen for components and growth factors that enhanced proliferation of bovine satellite cells 11 . Through multiple DOE rounds and response surface methodology for optimization, they developed a SFM containing fetuin, bovine serum albumin, FGF2, and an insulin-transferrin-selenium supplement 11 . In addition to testing known growth-promoting components, omics approaches can be effectively utilized to develop SFM. For instance, Lin and colleagues used intracellular metabolomics analyses to select candidate growth-promoting metabolites that could account for the differences in chicken fibroblasts’ growth profiles seen in two different basal media 15 . Using DOE, they achieved 40.72% higher cell growth by optimizing the concentrations of 28 candidate metabolites 15 . In another study, Messmer et al. employed a transcriptomic approach to identify surface receptors upregulated during myogenic differentiation induced by serum starvation 16 . By testing the corresponding ligands, they formulated a serum-free myogenic differentiation medium 16 . Next, Gomez Romero and Boyle’s review 17 highlights the use of systems biology and metabolomics tools to understand key metabolic pathways and genes 17 . These insights can be applied to media design and bioprocess optimization, potentially lowering cell-based meat production costs 17 . By understanding the metabolic and nutrients requirements of the human induced pluripotent stem cells, Lyra-Leite and colleagues managed to optimize and reduce the number of media components in B8 14 media down to 39 components 18 . While there have been successes in developing SFM for CM cell lines, studies reveal that nutrient requirements vary across different species and cell types 17 , 19 . This indicates that a one-size-fits-all SFM is unlikely to be effective for culturing multiple cell types. Additionally, variations in cellular metabolism at different stages of differentiation may necessitate optimizing SFM for both the cell growth and differentiation phases, requiring different formulations throughout the cultivated meat production process 20 . Therefore, SFM development pipelines must be robust enough to create tailored media formulations specific to the various cell lines and cell states in the CM industry. Serum-free media for cultivated meat Several cultivated meat companies, including Mosa Meat 8 , 16 , 21 , GOOD Meat 22 , Upside Foods 23 , Aleph Farms 24 , Believer Meat 25 , 26 , Vow 27 , and CellMeat 28 , have successfully developed SFM. Additionally, there are also a myriad of commercially available serum replacements for cultivated meat cell lines produced by various companies such as ClearX9 produced by Clear Meat 29 , Multus Biotechnology’s Proliferum M 30 , NouBio’s NouSerum 31 and microorganism-based growth medium supplement by Biftek.co 32 . Currently, several serum-free cultivated meat products have received regulatory approval in multiple countries. In January 2023, the Singapore Food Agency approved GOOD Meat’s serum-free cultivated chicken for production in Singapore 22 . In January 2024, Israel’s Ministry of Health approved Aleph Farms’ serum-free cultivated beef 24 , followed shortly by Singapore’s approval of Vow’s serum-free quail product 27 . Most recently, in July 2024, Meatly received regulatory clearance to produce its cultivated pet food in the United Kingdom 33 . Several SFM formulations are also published and summarized in Table 1 . A comparison of these formulations showed that DMEM/F-12 was commonly used as the basal media, as it combined the high nutrient content of DMEM with the diverse components of Ham’s F12. FGF2 was frequently employed as a growth factor, with insulin and transferrin also common media additives. Table 1 Published serum-free media formulation with concentrations for cultivated meat application A cost analysis of the published formulations at lab-scale is provided in Table 2 and Supplementary Table 1 - 6 . As expected, GFs and RPs account for the majority of the SFM cost, with basal media contributing the remainder. The analysis is based on pricing from life science vendors, but further cost reductions may be achievable through bulk purchasing or sourcing from B2B vendors specializing in the cultivated meat sector. Recently, Believer Meats demonstrated that a serum-free medium can be produced at a cost as low as USD $0.63 per liter 26 , by replacing albumin and optimising concentrations of media components based on the nutritional requirements of the cell. In the following sections, we will discuss potentials in reducing the cost contributions of growth factors, recombinant proteins, and basal media, as well as other strategies to enable more affordable SFM for cultured meat applications. Table 2 Published serum-free media formulation with lab scale cost analysis (SGD) per liter of SFM. The percentage contributions of basal media, proteins/peptides, and other components (mixture, steroids, hormones, salt, buffer, drug,) to the total cost are also shown. For detailed information on the calculation, refer to supplementary table 1 - 6 Advances to reduce the cost contribution by growth factors and recombinant protein Given that the cost of SFM is largely attributed to the use of GFs and RPs (Table 2 , Supplementary Table 1 - 6 ), much of the research focuses on reducing the use of GFs and RPs through genetically engineering cultivated meat cell lines or producing cheaper GFs and RPs. Genetic modification of cell lines to produce the required growth factors The idea of performing genetic modification of cell lines to remove the requirement for GFs in media started as early as 1992. Pietrzkowski et al. randomly integrated both human IGF1 and IGF1R into BALB/c3T3 mouse fibroblasts, finding that the resulting cells could grow in the absence of FBS 34 . Subsequently, another group randomly integrated human IGF1 and transferrin into CHO-K1 cells 35 . These modified cells could then grow in the absence of insulin and transferrin. In recent years, genetic modifications have also been attempted on cultivated meat cell lines. Stout and colleagues engineered immortalized bovine satellite cells (iBSCs) to express bovine FGF2 and human RASG12V genes under the control of the Dox-inducible Tet-on promoter 36 . This enabled a doubling time of 60 h, comparable to that of unmodified iBSCs supplemented with recombinant FGF that had a doubling time of 55 hours 36 . The engineered cells were able to proliferate in FGF2-free medium for multiple passages, although with reduced myotube formation during differentiation. Based on this work, Tufts University filed a patent claiming that modified bovine, piscine, galline, and porcine cells can grow in minimal media by ectopically expressing two or more growth factors, cytokines, or receptors (via ribosomal skipping sites) that promote cell growth, eliminating the need for exogenous growth factor supplementation 37 . Another patent was filed by Upside Foods, which used a PhiC31 integrase expression plasmid system to integrate FGF2, FGF receptors (including mutants), IGF1, and IGF1R under hEF1a promoter control into chicken fibroblasts 38 . The engineered cell lines were able to grow in growth factor-free media formulations, with expression of FGFRs surprisingly also reducing the requirement for IGF1 38 . While genetic modification of cell lines to remove the requirement for growth factors has the potential to reduce media costs, there are several considerations for the successful implementation of this approach. Firstly, there are consumer concerns over genetic modifications. Generally, there is a lack of longitudinal studies on perceptions of GMOs for consumption, with individual studies differing in survey methodology over time. Nonetheless, some studies suggest that opposition to GMOs may be softening. The European Food Safety Authority commissioned Kantar to conduct surveys in 28 EU Member States in April 2019 39 . Bearing in mind that questions are not directly comparable, 66% of respondents surveyed in 2010 were very or fairly worried about “genetically modified organisms found in food or drink,” compared to 27% of respondents ranking it in their top five concerns towards food in the 2019 survey. Similarly, in China, there may have been a shift in public perception, with 11.9% supporting genetically modified food in 2016 40 , increasing to 55% having a positive attitude towards GM foods in 2022 41 . Interestingly, in the 2019 Eurobarometer food safety survey only 4% of respondents ranked “Genome editing” in their top five concerns 39 , perhaps reflecting a preference for the minimal use of foreign DNA made possible in gene editing. Consequently, to increase consumer acceptance and reduce regulatory risk, groups are exploring the use of species-specific growth factors for genetic modification or performing reversible genetic modifications to remove foreign genetic material after the genes of interest have served their purpose. In a patent filed by Kent State University 42 , their technology proposes the use of TERT and cell-cycle genes such as CDK4, or even flavor-enhancing proteins like myoglobin, flanked by FRT or loxP recombination sites. After expansion to desired biomass or accumulation of myoglobin, these integrated cassettes can be removed via inducible or exogenously supplemented FLP or Cre recombinases. In another patent filed by Wildtype Inc 43 ., integrated trans-differentiation-relevant genes and a dox-inducible Cre (Tet-response element promoter + Cre) are flanked by loxP sites. After differentiation, both the trans-differentiation factors and Cre can be removed with the addition of doxycycline. Meanwhile, both previously-mentioned studies 37 , 38 exploring the ectopic expression of growth factors in CM cell-lines also investigated the use of species-specific growth factors, albeit under the control of foreign promoters. Lastly, while genetic modification might suffice for eliminating GF reliance, it may not be suitable for recombinant proteins that are required in large amounts such as albumin, which is required in mg/mL concentration ranges. In a previous study, Li et al. used Piggybac transposase to integrate several different secreted protein A-fusion proteins into adherent HEK293S GnTI-cells 44 . While as many as 15 copies of the DNA fragment could be inserted per cell, the highest-expressing clones for each protein could only attain 8–10 mg/L concentrations. Likewise, in suspension Freestyle 293-F shake-flask cultures, they were only able to get 5-30 mg/L concentrations 44 . Therefore, this approach might not be suitable for recombinant proteins required at high concentrations, for both adherent and suspension cultures. This highlights the need for a cheaper source of recombinant proteins and growth factors. Production of cheaper growth factors and recombinant proteins The recombinant production of these GFs/RPs is challenging and expensive because they require some level of post-translational modification. Consequently, despite Escherichia coli’s ubiquity as a recombinant protein expression system due to its ease of manipulation, speed, and cost, the commercial use of E. coli to directly produce soluble GFs without post-processing remains limited. After screening different GF genes, fusion partner combinations, and E. coli strains, Venkatesan and colleagues managed to produce a range of functionally active GFs, including FGF2, IGF1, PDGF-BB, and TGF-b1 and species-specific GFs in E. coli 45 . They also reported a significant reduction in the cost contribution of GFs in Essential 8 media down to 2%, from 86%, when using their own GFs. Similarly, Liu and colleagues also managed to express functional recombinant bovine FGF1 in E. coli, with final yields of 55 mg/L 46 . Molecular farming in plants is gaining popularity due to its environmental friendliness, ability to scale exponentially, and improvement in productivity. The technology involves inserting DNA into host plants and using specific portions of the plants, such as leaves or seeds, as expression hosts to produce GFs and RPs. GFs/RPs have been produced in a wide variety of plant species such as rice 47 , oilseed plants 48 , tobacco 49 and barley 50 . In interview, BioBetter claims to be able to produce insulin, transferrin, and FGF2 in tobacco plants and that their production cost of the protein is expected to reach $1 per gram of protein 49 . The cost of species-specific GFs produced for cultivated meat application were also generally lower as compared to PeproTech®’s growth factors from ThermoFisher Scientific (Table 3 ). Table 3 Price of recombinant growth factors for cultivated meat industry. (Cost were obtained from the various vendors’ website on 30 Oct 2024) Cell-free protein expression (CFPE) systems are another way to produce cheaper GFs and RPs. These allow for quick protein expression within 24 – 48 h of addition of genetic materials as compared to days or weeks in cell-based or plant-based expression systems. The production of the GFs and RPs is also performed in a controlled environment, allowing for different conditions such as pH, and temperature to be optimized. Several reviews on the use of CFPE expression systems exist 51 , 52 , 53 . Common CFPE systems include E. coli extract, yeast extract, wheat germ extract, tobacco BY-2 extract, insect cell extract and mammalian cell extract. LenioBio’s BY2-based platform can yield approximately 3 g/L of growth factors and recombinant proteins 48 . Similarly, Hitachi Zosen Corporation, in collaboration with NUProtein Co. Ltd, is utilizing wheat germ cell extract for the synthesis of GFs for cultivated meat applications 54 . Advances to reduce cost contribution of basal media through using cheaper raw materials To further reduce the cost of the SFM, the industry is also moving towards replacing pharmaceutical-grade media components with food-grade alternatives. A cost analysis conducted by Liz Specht highlighted that replacing basal medium components with bulk, food-grade, equivalents could reduce basal media cost by 77% 9 . Food-grade components are on average 82% cheaper compared to their reagent-grade alternatives at 1 Kg scale (Table 4 ). Pharmaceutical-grade materials are costlier due to their high purity and certification by rigorous quality standards, including additional purification and endotoxin testing not typically performed for food-grade materials 55 . Consequently, food-grade materials may have greater batch variability and contaminants, thus necessitating separate testing before use. For instance, Stellavato et al. found that of the ten chondroitin sulfate and glucosamine food supplements they tested, none of them contained their declared concentrations of both substances; all were contaminated with the structurally-similar keratan sulfate; and most were cytotoxic to their cell models 56 . Table 4 Price comparison between reagent-grade media component and food-grade media component. (Cost were obtained from Sigma-Aldrich website on 30 Oct 2024) Despite this, food-grade alternatives like plant hydrolysates are more cost-effective, still safe for human consumption, and nutrient-rich, containing bioactive compounds beneficial for animal cell culture. They are commonly used to reduce the amount of serum required 57 , and when used appropriately, can even replace traditional basal media by serving as a source of carbon and nitrogen 5 . The bioactive peptides found in hydrolysates can promote cell growth and offer additional benefits like anti-apoptotic, antioxidant, immunomodulatory properties, and potentially substituting growth factors 5 , 58 , 59 . Hydrolysates also lower storage and sterilization costs due to their heat stability 5 , 57 and can enhance the flavor and nutritional profile of cultivated meat 5 , 60 . Humbird also estimated that the use of plant hydrolysate will be able to reduce the cost of amino acids down to $2 per Kg of mixed amino acids 7 . Some cultivated meat companies have reported success in replacing pharmaceutical-grade media components with food-grade alternatives. For example, Mosa Meat, in partnership with Nutreco, replaced 99.2% of the basal cell feed by weight with food-grade components, while achieving cell growth comparable to pharmaceutical-grade media 61 . Similarly, Nutreco and Blue Nalu demonstrated that suspension bluefin tuna muscle-derived cells grew equally well in both food-grade and pharmaceutical-grade cell culture media 62 . A study by IntegriCulture Inc. demonstrated that mouse skeletal muscle-derived cells (C2C12) and bovine skeletal muscle-derived primary cells (BSMCs) can grow in food-grade DMEM 63 . Additionally, IntegriCulture Inc., together with JT Group, reduced 31 media components to 16 by replacing some amino acid components with yeast extract 64 . This food-grade I-MEM2.0 could support cell growth from various cell types and species, including bovine skeletal muscle-derived primary cells, duck liver-derived cells, and five different chicken primary cells 64 . There are already comprehensive reviews on the use of hydrolysates in animal cell culture, particularly those derived from non-animal sources such as soy, wheat, rice and yeast 57 , 58 , 59 , 65 . In the following section we will focus on advances in the use of hydrolysates from other sources, such as microalgae extracts and agricultural side streams for cultivated meat application. Microalgae Extracts Research on the use of microalgae extracts as cell culture supplements is gaining interest due to their environmental friendliness and high nutritional content, including proteins, fatty acids, trace elements, and vitamin B 66 , 67 . Extracts from Chlorella vulgaris have been shown to support the proliferation of embryonic bovine tracheal fibroblasts 68 and the proliferation and differentiation of primary bovine myoblasts 69 . Other microalgae are also being investigated. For instance, Chlorococcum littorale’s extracts have been found to promote cell growth in C2C12, 3T3, and Chinese hamster ovary (CHO) cell lines in serum-free environments 70 . Additionally, Cyanobacteria Anabaena sp. PCC 7120’s extract was used as a media supplement to cultivate mouse and quail muscle cells 71 . Next, Auxenochlorella pyrenoidosa protein extract – together with l-ascorbic acid, insulin, transferrin, selenium, and ethanolamine – was successfully used as FBS replacement for Carassius auratus (goldfish) muscle cell proliferation 72 . Despite their potential, the nutritional profiles of microalgae have not been thoroughly studied, with different algae species varying in their nutritional content. Future research could investigate other microalgae with Generally Recognized as Safe (GRAS) status, to evaluate their suitability as media supplements for cultivated meat applications 73 . Agricultural side streams To develop cost-effective media component replacements, researchers are also exploring the use of agricultural side streams for cellular agriculture. Efficient side stream valorization allows for the better utilization of agricultural byproducts, resulting in less waste and promoting a circular bioeconomy with lower overall monetary and environmental costs compared to FBS usage 74 , 75 . Various groups have attempted to use soybean meal as partial FBS replacement 76 , 77 , 78 . Kim and colleagues demonstrated that they could partially replace FBS with fermented soybean meal and edible insect hydrolysates for the cultivation of pig muscle stem cells 76 . Similarly, Teng and colleagues showed that okara extracts could partially replace FBS during the cultivation of C2C12 and immortalized porcine myoblasts 78 , although dose-dependent toxicity was observed. Additionally, rapeseed protein isolates from agricultural waste were found to be capable of replacing albumin used in Beefy-9 for the cultivation of bovine satellite cell cultures 79 . Hydrolysates from various animal-based food processing byproducts and yeast extract were also shown to support bovine skeletal muscle cells in terms of cell growth and function, with hydrolysates consisting of peptides 2 – 15 amino acids-long improving cell growth and increasing cellular metabolism 80 . Ultimately, while the utilization of side streams could result in less waste and lower cost, proper side stream management must be implemented to prevent unwanted contaminants. Considerations for using hydrolysates as food-grade media alternative Several issues must however be addressed before hydrolysates can be effectively used in cultivated meat production 5 . Variations in hydrolysate quality can arise from factors such as differences in raw materials, species, storage conditions, and processing methods 5 , 57 , 58 . Optimization of hydrolysates’ processing methods, well-defined and rigorous quality control testing methods, specifications for raw materials, qualitative and quantitative analysis of hydrolysate end-product are thus needed, to minimize batch-to-batch variation in cultivated meat production 58 , 65 , 81 . More studies will also need to be conducted to assess the effect of inter-batch variation of hydrolysates on cell mass, metabolism, and differentiation. Secondly, protein extracts may contain impurities such as heavy metals, pesticides, cytotoxic and allergenic components 60 , 75 . Group 2B compounds, possibly carcinogenic compounds such as free and esterified forms of 3-monochloro-1,2-propanediol and 1,3-dichloro-1,2-propanol, are reported to be found in acid-hydrolyzed vegetable proteins 60 . Furthermore, the use of nut-based hydrolysates might have immunological cross-reactivity in peanut-allergic consumers 60 . To address these safety concerns, hydrolysates can be produced from GRAS organisms or traditional crops/foods with a history of human consumption. Future studies should also investigate the accumulation of impurities in cultivated meat cells and their impact on human consumption. Finally, while hydrolysates can support cell growth, they still lack some nutrients compared to FBS. Identifying and supplementing these missing nutrients is essential to achieving better growth than FBS-containing cultures and to fully replace FBS and current basal media 71 . Effects of hydrolysates as serum replacement are also cell-line dependent 57 . More research is needed for industry-wide adoption of hydrolysates as pharmaceutical-grade media replacements. Increasing the longevity of the serum-free media for cost reduction Another way to reduce the cost of media, and labour costs associated with media-change operations, will be to increase the usability of the media for a longer period. A comprehensive review was published by Yang et al. on preventing waste buildup through genetic engineering techniques, cell culture strategies, affinity removal methods, biocatalytic methods, and electrochemical methods 82 . In this section, we will discuss preventing waste buildup through the optimization of media components, improving GF stability and other media recycling techniques. Optimization of media components Ammonia, a potent inhibitor of cell growth, is produced during glutamine metabolism. To reduce ammonia production during the proliferation and adipogenesis of fibro-adipogenic progenitors (FAPs), Hubalek et al. developed novel serum-free media for both proliferation and differentiation by replacing GlutaMAX with non-ammoniagenic compounds like α-ketoglutarate (aKG), glutamate, and pyruvate, which are involved in the TCA cycle or glutaminolysis 83 . They showed that pyruvate and aKG led to comparable cell growth rates with no ammonia produced during short-term proliferation 83 . FAPs were also able to maintain their differentiation capacity when Glutamax was replaced with aKG, pyruvate and glutamate, with a 2.1-fold increase in adipogenic capacity in cells grown and differentiated in non-ammoniagenic media 83 . The substitution of glutamine by other components to reduce ammonia buildup was also successfully demonstrated in other commercial cell lines 84 , 85 . Replacing glutamine with TCA intermediates like aKG, citric acid, and succinic acid in recombinant CHO cell cultures resulted in similar growth rates after a lag phase and significantly reduced ammonia production 84 . Cell growth of various mammalian cell types Madin-Darby canine kidney cells, Baby Hamster kidney fibroblasts and CHO cells was also supported for at least 19 passages in media containing pyruvate as a substitution for glutamine, with no ammonia accumulation 85 . Other than ammonia production, media optimization could also be performed to reduce production of lactate 86 , 87 , another potent inhibitor of cell growth that arises from glycolysis and amino acid catabolism. To that end, our lab demonstrated that using maltose as an alternative energy source to glucose for CHO cells and HEK293 cells resulted in lower levels of lactate, which could potentially improve cell and protein productivity 88 . However, other disaccharides failed to sustain cell growth 88 . Similarly, Buchsteiner et al. were able to reduce lactate production, without adverse effects, in CHO cells by supplementing the media with dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase 89 . Another way to prolong the media lifespan is to stabilize the growth factors used. For example, the common GF supplement, FGF2, was found to be stabilized and prevented from heat-induced aggregation by addition of its known cofactor, heparin 90 . Alternatively, thermostable FGF2 mutants, such as FGF2-G3 with nine point mutations to increase its half-life from 10 h to 20 days 91 , have successfully been used in the culture of induced pluripotent stem cells to lower media change frequencies 14 . Similarly, thermostable mutants of FGF1 92 and FGF7 93 have been reported, although work on other GFs such as IGF1 and EGF is lacking. Media Recycling Although the concept of media recycling to reduce media costs first started in 1977 94 , 95 , Believer Meats is the only cultivated meat company to implement spent media recycling technology to date 96 . In 2017, Yaakov Nahmias, Believer Meats’ founder, submitted a patent application for a closed-loop perfusion bioreactor system 96 . This system enables the recycling of media by guiding it through a dialyzer that filters out harmful metabolites, retaining large proteins such as albumin and replenishes nutrients before circulating back to the tissue growth chambers where cells are cultivated Media recycling has also been applied in areas beyond cultivated meat production. Masahiro Kino-oka’s team proposed a dialysis setup to remove lactate and to recycle autocrine factors and GFs for the culturing of human induced pluripotent stem cells in suspension 97 . With this setup, cells could be maintained with lower concentrations of supplemented TGF-β1, insulin, transferrin, L-ascorbic acid, and selenium. In another study by Akiko Ogawa’s team, media recycling was achieved by passing spent media through an affinity protein G column, concentrating it by ultrafiltration, then diluting it with fresh basal medium 98 . In doing so, they saved 67% of the complete media for growing IgG1 antibodies producing hybridoma cells. In a similar study by Lin’s team, the permeate from perfusion CHO cultures used for monoclonal antibody production was successfully recycled by passing sequentially through a Protein A column and cation exchange depth filter to remove process impurities, then mixed with concentrated media 99 . They, however, cautioned that since their recycled stream required mixing with media concentrate and subsequent osmolality balancing to retain media depth and cell proliferation, only water was effectively being saved, with little material cost benefits 99 . Microalgae are also being explored for renewing spent mammalian culture media by removing waste like ammonia and lactate and replenishing depleted nutrients via microalgae extract supplementation. For instance, Chlorococcum littorale removes over 90% of ammonia while restoring glucose and amino acids 100 . To enhance this process, Yuji and his team used recombinant Synechococcus sp. PCC 7002 that can utilize both ammonia and lactate 101 . However, nutrient levels in microalgae extracts can vary, requiring adjustments to the microalgae extract added and growth factors need supplementation after several media renewal cycles 100 , 101 . Conclusion In conclusion, cultivated meat production holds immense promise for addressing global food security and sustainability challenges. However, the current production process remains expensive, primarily due to the high cost of SFM. Achieving price parity with conventional meat requires the development of scalable, low-cost culture media. This review has explored the current technologies and opportunities for creating cost-effective SFM tailored to cultivated meat production. Key strategies include reducing the costs associated with growth factors and recombinant proteins and lowering the expense of basal media by utilizing more affordable raw materials. Additionally, innovative approaches such as media recycling and waste reduction were discussed as alternative cost-saving measures. While this review focused on economic aspects, it is also important to recognize these strategies could also reduce the environmental impact of cultivated meat productions. Other critical factors in media development, such as the impact of culture media on the organoleptic and nutritional qualities of cultivated meat products, were beyond the scope of this review but remain vital areas for future research. Data Availability Acknowledgements The work is supported by National Research Foundation (NRF), Agency for Science, Technology and Research (A*STAR) and Singapore Food Agency Singapore Food Agency (SFA) under Singapore Food Story R&D Programme grants; H20H8a0003, W22W3D0004 and W23W2D0009. We would also like to acknowledge administrative and funding support from Bioprocessing Technology Institute, Agency for Science, Technology and Research (A*STAR). Author information Authors and Affiliations Bioprocessing Technology Institute (BTI), Agency for Science, Technology and Research (A*STAR), 20 Biopolis Way, #06-01 Centros, Singapore, 138668, Republic of Singapore Jun Ping Quek, Azra Anwar Gaffoor, Yu Xuan Tan, Tessa Rui Min Tan, Yu Feng Chua, Dawn Sow Zong Leong, Alif Sufiyan Ali & Say Kong Ng Authors Additional information Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Supplementary information Rights and permissions Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
UPSIDE Foods Frequently Asked Questions (FAQ)
When was UPSIDE Foods founded?
UPSIDE Foods was founded in 2015.
Where is UPSIDE Foods's headquarters?
UPSIDE Foods's headquarters is located at 804 Heinz Avenue, Berkeley.
What is UPSIDE Foods's latest funding round?
UPSIDE Foods's latest funding round is Series C.
How much did UPSIDE Foods raise?
UPSIDE Foods raised a total of $610.85M.
Who are the investors of UPSIDE Foods?
Investors of UPSIDE Foods include SOSV, IndieBio, SoftBank, Bill Gates, Kimbal Musk and 32 more.
Who are UPSIDE Foods's competitors?
Competitors of UPSIDE Foods include Planted, Uncommon, Prolific Machines, Finless Foods, Mosa Meat and 7 more.
Loading...
Compare UPSIDE Foods to Competitors

SuperMeat is a food tech company focused on the production of cultivated meat, particularly chicken, using a process that claims to be sustainable and animal-friendly. The company offers cultivated meat products that provide nutritional security, aim to reduce carbon emissions, and improve food safety. SuperMeat operates 'The Chicken', a facility where visitors can observe the production of cultivated meat and experience meals created by their team of food engineers and chefs. It was founded in 2015 and is based in Tel Aviv, Israel.

Believer Meats focuses on the production of cultivated meat within the food technology sector. It offers real, non-GMO meat grown from animal cells, providing a cruelty-free, antibiotic-free, and environmentally friendly alternative to conventionally farmed meat. The company primarily serves consumers looking for healthier and sustainable meat options. Believer Meats was formerly known as Future Meat Technologies. It was founded in 2018 and is based in Rehovot, Israel.

Aleph Farms is a food technology company that specializes in cell-grown meat. It grows beef steaks from non-genetically engineered and non-immortalized cells isolated from a living cow without slaughtering the animals. The company was founded in 2017 and is based in Rehovot, Israel.

Mosa Meat focuses on beef alternatives within the food industry. The company specializes in producing cultured beef burgers, utilizing cell-based meat technology to create real beef without the need to raise and slaughter animals. It primarily serves the food industry. The company was founded in 2016 and is based in Maastricht, Netherlands.

Prime Roots focuses on reimagining protein by offering plant-based meat alternatives in the food industry. The company's products replicate the taste and texture of traditional meats using koji, a sustainable and environmentally friendly protein source. Prime Roots caters to a variety of eaters looking for better meat options without the environmental costs associated with animal proteins. Prime Roots was formerly known as Terramino Foods. It was founded in 2017 and is based in Berkeley, California.

Rebellyous Foods specializes in the production of plant-based chicken alternatives within the food industry. Its main offerings include a variety of nuggets, patties, and tenders designed to replicate the taste and texture of traditional chicken products. The company primarily serves the retail, food service, and educational sectors of food options. It was formerly known as Seattle Food Tech. It was founded in 2017 and is based in Seattle, Washington.
Loading...