
Carmot Therapeutics
Founded Year
2008Stage
Acquired | AcquiredTotal Raised
$374.88MValuation
$0000About Carmot Therapeutics
Carmot Therapeutics is a clinical-stage biotechnology company. It is focused on the discovery and development of disease-modifying therapies for people living with metabolic diseases including obesity and diabetes. The company utilizes chemotype evolution, a pioneering drug discovery platform, to identify novel incretin receptor signaling targets and develop a broad pipeline of therapeutics. It was founded in 2008 and is based in Berkeley, California. In December 2023, Carmot Therapeutics was acquired by Roche at a valuation in the range of $2.7B and $3.1B.
Loading...
Loading...
Research containing Carmot Therapeutics
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Carmot Therapeutics in 2 CB Insights research briefs, most recently on Nov 27, 2024.
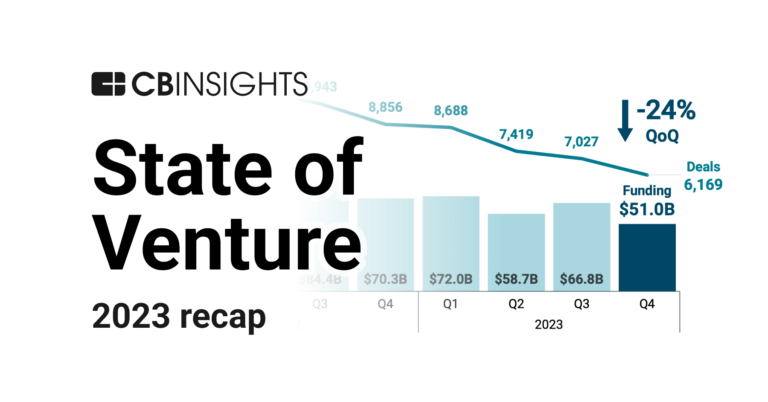
Jan 4, 2024 report
State of Venture 2023 ReportCarmot Therapeutics Patents
Carmot Therapeutics has filed 10 patents.
The 3 most popular patent topics include:
- diabetes
- g protein coupled receptors
- rare diseases
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
8/13/2019 | 2/7/2023 | Rare diseases, Proteins, Autosomal recessive disorders, Human proteins, Transcription factors | Grant |
Application Date | 8/13/2019 |
|---|---|
Grant Date | 2/7/2023 |
Title | |
Related Topics | Rare diseases, Proteins, Autosomal recessive disorders, Human proteins, Transcription factors |
Status | Grant |
Latest Carmot Therapeutics News
Dec 2, 2024
Posted on DelveInsight’s “Obesity Market Insights, Epidemiology and Market Forecast–2034” report delivers an in-depth understanding of obesity, historical and forecasted epidemiology as well as the obesity market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan. Key Takeaways from the Obesity Market Report In November 2024:- Carmot Therapeutics Inc.- A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multi-Center Phase 2 Study to Evaluate the Efficacy, Safety, and Tolerability of Once-Weekly CT-388 Administered Subcutaneously for 48 Weeks to Participants Who Are Overweight or Obese with Type 2 Diabetes Mellitus. This is a multi-center, randomized, double-blind, placebo-controlled, parallel group dose-finding study to evaluate the efficacy and safety of CT-388 at low, middle, and high doses in participants who are overweight or obese with Type 2 diabetes mellitus (T2DM). In November 2024:- Eli Lilly and Company- A Phase 3, Randomized, Double-Blind Study to Evaluate the Efficacy and Safety of Retatrutide Compared to Tirzepatide in Adults Who Have Obesity. The main purpose of this study is to evaluate the efficacy and safety of retatrutide compared to tirzepatide in adults who have obesity. The study will last about 89 weeks. As per DelveInsight’s estimates, the United States accounted for the highest number of total prevalent cases of Obesity among the 7 MM in 2023. DelveInsight’s consultant estimates that adult patients constituted the maximum number of cases of obesity patients seeking help in 2023. According to the findings, treatment rate for children was found to be less than that of adults across countries. Among EU4 and the UK, the highest number of treated cases of obesity in adults was observed in the United Kingdom in 2023, which is followed by Germany. The leading Obesity Companies such as Rhythm Pharmaceuticals, Boehringer Ingelhium, D&D Pharmatech, ProQR Therapeutics, Nano Precision Medical, Bukwang Pharmaceutical, Caliway Biopharmaceutics, Yuhan, Terns Pharmaceuticals, BioRestorative Therapies, SCOHIA PHARMA, Click Therapeutics, Hanmi Pharmaceuticals, Novo Nordisk, Empros Pharma, Carmot Therapeutics, Eli Lilly and Company, and others Promising Obesity Therapies such as IMCIVREE (setmelanotide), ZEPBOUND (tirzepatide), Semaglutide oral, Survodutide (BI 456906), DD03, AX-0601, NPM 139, BK-1701, CBW-520, YH34160, TERN-601, Thermostem, SCO-267, CT-181, HM15136, NNC0480-0389, EMP-16, CT-868, Semaglutide, and others IMCIVREE (setmelanotide): Rhythm Pharmaceuticals Imcivree is a melanocortin 4 (MC4) receptor agonist indicated for chronic weight management in adult and pediatric patients 6 years of age and older with obesity. In November 2020, Rhythm Pharmaceuticals announced the US FDA approval of Imcivree as the first-ever therapy for chronic weight management in patients with obesity due to POMC, PCSK1, or LEPR deficiency. Earlier, in May 2017, FDA expanded the Breakthrough Therapy Designation to disorders involving genetic defects upstream of the melanocortin-4 receptor in the leptin-melanocortin pathway. ZEPBOUND (tirzepatide): Eli Lilly and Company Tirzepatide is a once-weekly glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonist that integrates the actions of both incretins into a single novel molecule. In November 2023, the US FDA approved Eli Lilly and Company’s ZEPBOUND (tirzepatide) injection, the first obesity treatment of its kind that activates both GIP and GLP-1 hormone receptors. ZEPBOUND is indicated for adults with obesity (with a BMI of 30 kg/m2 or greater), or those who are overweight (with a BMI of 27 kg/m2 or greater) and also have weight-related medical problems such as hypertension, dyslipidemia, Type II diabetes mellitus, obstructive sleep apnea or cardiovascular disease, to lose weight and keep it off. Semaglutide oral: Novo Nordisk Oral semaglutide is approved as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes in the US, EU and Japan under the trade name, RYBELSUS. It is an oral GLP-1 RA, an analogue of the naturally occurring hormone GLP-1. Currently, the company is developing the candidate for obesity. The candidate is being evaluated in various Phase III trials (OASIS 1, OASIS 2, OASIS 3 and OASIS 4) to assess the safety and efficacy of the drug in subjects with overweight or obesity. Survodutide (BI 456906): Boehringer Ingelhium Survodutide (BI 456906) is a GCGR/GLP1 dual agonist being developed by Boehringer Ingelhium. GLP1 agonism is expected to lower body weight and provide glucose control. Both receptors are critical to controlling metabolic functions. The compound leverages the known effects of the natural gut hormone oxyntomodulin, which has been shown to decrease food intake and increase energy expenditure in humans as well as the established effects of GLP1 agonism on both glucose control and body weight. The dual agonist BI 456906 has potential as a new, once-weekly treatment that may offer therapeutically relevant benefits compared to currently available treatments. Coverage- 7MM Obesity Companies- Rhythm Pharmaceuticals, Boehringer Ingelhium, D&D Pharmatech, ProQR Therapeutics, Nano Precision Medical, Bukwang Pharmaceutical, Caliway Biopharmaceutics, Yuhan, Terns Pharmaceuticals, BioRestorative Therapies, SCOHIA PHARMA, Click Therapeutics, Hanmi Pharmaceuticals, Novo Nordisk, Empros Pharma, Carmot Therapeutics, Eli Lilly and Company, and others Obesity Therapies- IMCIVREE (setmelanotide), ZEPBOUND (tirzepatide), Semaglutide oral, Survodutide (BI 456906), DD03, AX-0601, NPM 139, BK-1701, CBW-520, YH34160, TERN-601, Thermostem, SCO-267, CT-181, HM15136, NNC0480-0389, EMP-16, CT-868, Semaglutide, and others. Obesity Therapeutic Assessment: Obesity Current marketed and Obesity Emerging Therapies Obesity Market Dynamics: Obesity Market drivers and Obesity Market Barriers
Carmot Therapeutics Frequently Asked Questions (FAQ)
When was Carmot Therapeutics founded?
Carmot Therapeutics was founded in 2008.
Where is Carmot Therapeutics's headquarters?
Carmot Therapeutics's headquarters is located at 740 Heinz Avenue, Berkeley.
What is Carmot Therapeutics's latest funding round?
Carmot Therapeutics's latest funding round is Acquired.
How much did Carmot Therapeutics raise?
Carmot Therapeutics raised a total of $374.88M.
Who are the investors of Carmot Therapeutics?
Investors of Carmot Therapeutics include Roche, The Column Group, RA Capital Management, Deep Track Capital, Willett Advisors and 18 more.
Who are Carmot Therapeutics's competitors?
Competitors of Carmot Therapeutics include Echelon Biosciences, National Disease Research Interchange, DiscoveryBioMed, InterveXion Therapeutics, Cognition Therapeutics and 7 more.
Loading...
Compare Carmot Therapeutics to Competitors
Active Pass is a Canadian biotechnology company that is engaging in genomic-based drug discovery and is developing drugs that inhibit ABC transporters. ABC or (ATP binding cassette) is a term that describes the interconnected group of molecular engines or pumps that function as a transportation system which moves compounds across the biological membranes. These ABCs are very closely associated with human diseases like Alzheimer's disease, diabetes, asthma, and hypertension. The Active Pass management team is an accomplished group with experience in many aspects of drug discovery and development. Besides employing full time pharmaceutical development professionals, Active Pass has made wise use of a group of several key partnerships with different pharmaceutical groups. The company cultivates these partnerships in order to access new capabilities or expertise to advance the company's own technologies. More specifically, these partnerships include the Vaccines and Infectious Diseases Organization as well as the National Institute of Health). The company hopes to add new personnel to cover emerging areas of emphasis like clinical and regulatory affairs, business development and finance as they grow.
Medicor Biosciences, founded in 2006 is a development-stage company focused on drug discovery for combating cancer. The founding scientists are leaders in a class of anti-hormone drugs that are designed to block the relaxin hormone cellular signaling pathways in a number of different cancer types. Using the company's Relaxofen technology platform, therapeutic candidates are generated by engineering the natural relaxin peptide to display antagonistic properties. Through pre-clinical development and clinical trials, Medicor Biosciences plans to develop and potentially license the technology so that the therapeutics can be available for cancer patients as an anti-hormone therapy to impair the growth and spread of the disease.
Trana Discovery is a drug discovery technology company focused on developing new treatments for infectious diseases. The company offers a proprietary technology platform that assists partners in identifying potential drug candidates for bacterial, viral, and fungal infections. This technology is based on the inhibition of organism-specific transfer RNA (tRNA), which is crucial for the pathogen's propagation. It was founded in 2000 and is based in Cary, North Carolina.
InterveXion Therapeutics is a clinical-stage biotechnology company focused on developing therapies for methamphetamine use disorder. The company's main offerings include short and long-acting monoclonal antibodies and vaccines designed to bind methamphetamine and prevent its entry into the central nervous system. InterveXion primarily serves the healthcare sector, with a focus on addiction treatment and overdose management. It was founded in 2002 and is based in Little Rock, Arkansas.
GNI is a global pharmaceutical development company specializing in gene regulatory network methodologies within the biotechnology sector. The company offers pharmaceutical agents and meal replacements, focusing on the development of new drugs with increased efficacy and reduced side effects through advanced gene expression analysis and systems biology. GNI primarily serves sectors related to infectious diseases, cancer, and cardiovascular health. It is based in Japan.

Melior Discovery focuses on in vivo pharmacology and phenotypic screening within the biopharmaceutical industry. The company offers preclinical drug development services, including efficacy data collection, pharmacokinetic evaluations, and specialized animal model testing. Melior Discovery primarily serves the biopharmaceutical sector, providing tools and services for drug repositioning and indications discovery. It was founded in 2005 and is based in Exton, Pennsylvania.
Loading...