Caris Life Sciences
Founded Year
2008Stage
Loan | AliveTotal Raised
$1.714BValuation
$0000Last Raised
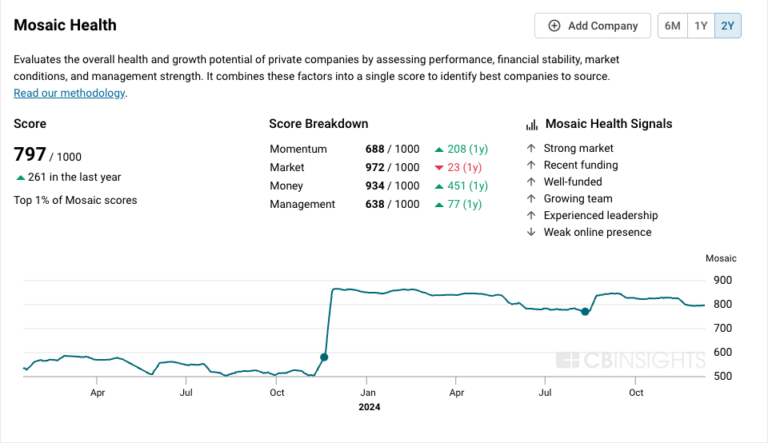
$400M | 2 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-16 points in the past 30 days
About Caris Life Sciences
Caris Life Sciences provides molecular profiling services, including DNA and RNA sequencing, to supply oncologists with information about cancer at the molecular level, facilitating personalized treatment plans. The company also offers biopharmaceutical solutions, assisting in the discovery, clinical development, and commercialization of new cancer therapies. It was founded in 2008 and is based in Irving, Texas.
Loading...
ESPs containing Caris Life Sciences
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The liquid biopsy cancer screening market offers a non-invasive alternative to traditional tissue biopsies for detecting cancer. This market includes various solutions such as circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) tests, which can detect cancer at an early stage and monitor treatment effectiveness. The liquid biopsy method is less invasive, faster, and more cost-effectiv…
Caris Life Sciences named as Outperformer among 15 other companies, including Exact Sciences, QIAGEN, and Veracyte.
Loading...
Research containing Caris Life Sciences
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Caris Life Sciences in 4 CB Insights research briefs, most recently on Dec 11, 2024.
Aug 22, 2022 report
State of Biopharma Tech Q2’22 Report
May 26, 2022
Where are the next US tech hubs?Expert Collections containing Caris Life Sciences
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Caris Life Sciences is included in 3 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,261 items
Precision Medicine Tech Market Map
160 items
This CB Insights Tech Market Map highlights 160 precision medicine companies that are addressing 9 distinct technology priorities that pharmaceutical companies and healthcare providers face.
Oncology Tech
463 items
This collection includes companies applying technology to cancer care, diagnosis, and treatment. Examples include vendors offering cancer detection and diagnosis, oncology clinical decision support, real-world data, and AI oncology drug discovery.
Caris Life Sciences Patents
Caris Life Sciences has filed 1 patent.
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
3/6/2018 | 3/9/2021 | Monoclonal antibodies, Molecular biology, Clusters of differentiation, Immunology, Biotechnology | Grant |
Application Date | 3/6/2018 |
|---|---|
Grant Date | 3/9/2021 |
Title | |
Related Topics | Monoclonal antibodies, Molecular biology, Clusters of differentiation, Immunology, Biotechnology |
Status | Grant |
Latest Caris Life Sciences News
Feb 11, 2025
News provided by Share this article Share toX A large, blood-based profiling study accurately identified clonal hematopoiesis (CH) variants, effectively decreasing clinical false positives missed by other plasma only approaches IRVING, Texas, Feb. 11, 2025 /PRNewswire/ -- Caris Life Sciences ® (Caris), a leading next-generation AI TechBio company and precision medicine pioneer, today announced the publication of " Characterization of plasma cell-free DNA variants as of tumor- or clonal hematopoiesis-origin in 16,812 advanced cancer patients ," in Clinical Cancer Research. Utilizing Caris Assure ™ blood-based profiling, the study shows how CH-associated variants, which are highly prevalent in cancer patients, can often overlap with therapeutic targets. The findings underscore the importance of distinguishing CH- from tumor-derived mutations to improve diagnostic accuracy and guide appropriate cancer therapy. The study characterized plasma cell-free DNA (cfDNA) variants of tumor, CH or germline origin in 16,812 patients with advanced cancer using Caris Assure, which sequences both the plasma and buffy coat using a proprietary whole exome and whole transcriptome assay. CH occurs when a mutation-carrying hematopoietic stem cell produces blood cells with the same mutation. As people age, these mutations build up in the blood and can be confused with cancer cells during blood tests, leading to false positives that complicate treatment decisions. Key findings include: CH prevalence: CH variants were detected in 42.3% of patients, with 76% of CHEK2, 66.5% of BRCA2 (66.5%), and 58.6% of BRCA1 somatic-derived variants arising from CH. Age correlations: CH rates increased with age from 20% in patients ages 65-69 years to 50% in patients ages 80 and older. Clinical impact: High CH rates were found in otherwise actionable genetic variants associated with PARP inhibitor (PARPi) therapies in breast, ovarian, pancreatic, prostate and endometrial cancer, with nearly 6% of prostate cancer patients having a CH mutation in a PARPi-associated gene. Without accurate CH classification, these patients could be inappropriately directed toward PARPi therapy. "Without accurate CH classification, oncologists risk recommending therapies based on mutations that do not come from the tumor," said George W. Sledge, Jr., MD , EVP and Chief Medical Officer of Caris and study author. "Caris Assure is the only blood profiling assay on the market that uses sequencing rather than algorithmic estimations to accurately determine the mutational origin, to our knowledge." "This study, the largest of CH in cfDNA to date, sets a new benchmark for liquid biopsy accuracy and cancer care," said Caris President and study author David Spetzler, MS, PhD, MBA . "By leveraging paired plasma and buffy coat analysis, Caris Assure accurately identifies CH variants, providing clinicians with precise insights into tumor biology and ensuring that every treatment decision is based on the most reliable information available." Detailed Scientific Information At least one CH variant was identified within reportable clinical genes in 42.3% of the 16,812 patients. A total of 41,380 reportable somatic mutations were detected in the plasma, of which 11,639 (27.8%) were classified as CH. More than three-quarters of CHEK2 variants were of CH origin, as were 66.5% of BRCA2, 58.6% of BRCA1, 46.2% of ATM, 7.8% of NRAS, 6.1% of BRAF, 2.6% of EGFR and 2.1% of KRAS variants. For patients ages 65-69 years, the median proportion of CH classification was 20%, whereas it was 33% for patients ages 70-74, 33% for ages 75-79 and 50% for ages 80 and older. Prevalence of CH also varied by cancer type, from 13.6% in uveal melanoma to 61.5% in uterine serous carcinoma. High rates of CH variants were detected in what would be otherwise druggable targets in many cancer types typically treated with PARPi therapy, including breast, ovarian, pancreatic, prostate and endometrial cancers. These findings point to CH as a critical source of false positive variants for biomarker-directed PARPi therapy, highlighting the need for thorough CH classification during liquid biopsy to recommend therapies appropriately. Clinicians should carefully interpret liquid biopsy results used to determine PARPi eligibility and avoid relying on these results unless the assay specifically identifies and subtracts CH variants. The study was performed in collaboration with members of the Caris Precision Oncology Alliance ™ (Caris POA), which includes 96 cancer centers, academic institutions, research consortia and healthcare systems, including 47 NCI-designated cancer centers, collaborating to advance precision oncology and biomarker-driven research. Caris and POA members work together to establish and optimize standards of care for molecular testing through innovative research focused on predictive and prognostic markers that can improve clinical outcomes for cancer patients. About Caris Life Sciences Caris Life Sciences® (Caris) is a leading next-generation AI TechBio company and precision medicine pioneer that is actively developing and delivering innovative solutions to revolutionize healthcare and improve the human condition. Through comprehensive molecular profiling (Whole Exome and Whole Transcriptome Sequencing) and the application of advanced AI and machine learning algorithms, Caris has created the large-scale, multimodal database and computing capability needed to analyze and further unravel the molecular complexity of disease. This convergence of sequencing power, big data and AI technologies provides a differentiated platform to deliver the next generation of precision medicine tools for early detection, diagnosis, monitoring, therapy selection and drug development. Caris was founded with a vision to realize the potential of precision medicine in order to improve the human condition. Headquartered in Irving, Texas, Caris has offices in Phoenix, New York, Cambridge (MA), Tokyo, Japan and Basel, Switzerland. Caris or its distributor partners provide services in the U.S. and other international markets. To learn more, please visit CarisLifeSciences.com . Caris Life Sciences Media:
Caris Life Sciences Frequently Asked Questions (FAQ)
When was Caris Life Sciences founded?
Caris Life Sciences was founded in 2008.
Where is Caris Life Sciences's headquarters?
Caris Life Sciences's headquarters is located at 750 West John Carpenter Fwy, Irving.
What is Caris Life Sciences's latest funding round?
Caris Life Sciences's latest funding round is Loan.
How much did Caris Life Sciences raise?
Caris Life Sciences raised a total of $1.714B.
Who are the investors of Caris Life Sciences?
Investors of Caris Life Sciences include OrbiMed Advisors, Braidwell, Sixth Street Partners, Neuberger Berman, Highland Capital Management and 15 more.
Who are Caris Life Sciences's competitors?
Competitors of Caris Life Sciences include Syapse and 6 more.
Loading...
Compare Caris Life Sciences to Competitors

Genialis is an RNA biomarker company that focuses on advancing translational research and cancer drug development within the healthcare sector. The company specializes in discovering RNA biomarkers using machine learning, which aids in positioning oncology drugs, stratifying clinical trials, and developing diagnostic tests to predict patient responses and guide treatment decisions. Genialis collaborates with pharmaceutical and diagnostics companies to deliver precision medicine solutions that aim to improve clinical outcomes. It was founded in 2015 and is based in Boston, Massachusetts.

GenomOncology is a software company that focuses on cancer care through data analysis. The company provides software solutions that process healthcare data into treatment options and insights, aimed at supporting oncology workflows. GenomOncology serves healthcare institutions and molecular labs, offering tools for data enablement, pathology, decision support, and analytics. It was founded in 2012 and is based in Cleveland, Ohio.

OncoHealth is a digital health company that provides a platform for oncology value management and virtual cancer care. The platform includes nursing, mental health, nutrition, and resource navigation services for patients and healthcare providers. OncoHealth serves health plans, employers, providers, and patients in the cancer care ecosystem. It was founded in 2009 and is based in Atlanta, Georgia.

CureMatch is a healthcare technology company that specializes in precision oncology and digital decision support solutions for cancer treatment. The company offers services that analyze genomic biomarkers to match patients with personalized cancer treatments, utilizing AI algorithms to rank combination therapy options. CureMatch primarily serves oncologists and cancer treatment centers seeking to implement precision medicine strategies. It was founded in 2015 and is based in San Diego, California.

CancerIQ is a platform that focuses on cancer prevention and early detection within the healthcare sector. The company provides software and services that assist in risk assessment, patient navigation, care plan management, and analytics for healthcare providers. CancerIQ's solutions serve oncology and genetics, breast centers, and preventive care sectors, allowing for cancer risk programs and integration with clinical workflows. It was founded in 2013 and is based in Chicago, Illinois.

Medidata specializes in providing a unified platform for clinical research within the life sciences sector. The company offers a range of products and solutions designed to streamline clinical trials, including data management, clinical operations, and patient engagement technologies. Medidata's platform serves various sectors, including biopharma, medical device companies, and academic research organizations. It was founded in 1999 and is based in New York, New York.
Loading...